Journal of Clinical Neuroscience and Rehabilitation
Neurological Disorders and Innovations in Targeted Drug Delivery
Sandip Prasad Tiwari1 and Mahendra Kumar Sahu2*
1Faculty of Pharmacy, Kalinga University, Naya Raipur, CG, India
2Research Scholar, Faculty of Pharmacy, Kalinga University, Naya Raipur, CG, India
Cite this as
Tiwari SP, Sahu MK. Neurological Disorders and Innovations in Targeted Drug Delivery. J Clin Neurosci Rehabil. 2025;1(1):004-020. Available from: 10.17352/jcnr.000002Copyright Licence
© 2025 Tiwari SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Chronic stress is known to have significant effects on brain function and structure, particularly in regions such as the hippocampus, which plays a crucial role in memory and emotion regulation. This neuroscience study aimed to investigate the relationship between neuronal disorders, pathogenesis, and drug delivery techniques. Researchers utilized a combination of behavioral assessments, neuroimaging techniques, and molecular analyses to examine the impact of stress on the brain. Animal models were subjected to various stressors over time, with their brain activity monitored using EEG and MRI scans. Post-mortem analyses further assessed gene expression changes and neurogenesis within the hippocampus. Control groups were included to compare the effects of stress on brain function. The results demonstrated that sleep deprivation significantly impairs cognitive functions such as memory, attention, and decision-making. These findings emphasize the critical role of sleep in maintaining optimal brain health and cognitive performance, reinforcing the importance of adequate rest for overall neurological well-being.
Introduction
Neuroscience is a multidisciplinary field that explores the structure and function of the nervous system, including the brain, spinal cord, and peripheral nerves. It encompasses a wide range of disciplines, such as biology, psychology, physics, and computer science, to understand how the brain and nervous system work. By examining neurons, synapses, neurotransmitters, and specific brain regions, neuroscientists seek to understand the neural mechanisms underlying cognition and behavior, and neurological disorders. Advances in neuroscience have led to groundbreaking discoveries in areas such as neuroplasticity, brain development, and the neural basis of consciousness [1]. The field continues to deepen our understanding of the brain’s structural and functional complexity and has far-reaching implications for medicine, psychology, and artificial intelligence. Trends in neuronal disorder prevalence over time reflects a significant shift in our understanding and management of these conditions over time. In the past, neuronal disorders were often stigmatized and poorly understood, which historically resulted in limited treatment options and care disparities and societal misconceptions. However, with advancements in neuroscience and medical research, our understanding of neuronal disorders has greatly improved. There is now broader scientific understanding of the underlying mechanisms of these conditions, leading to earlier diagnosis, better treatment strategies, and improved quality of life for patients. Additionally, there has been a greater emphasis on destigmatizing neuropsychiatric conditions and promoting early detection about the importance of early intervention and support for individuals with neuronal disorders [2]. While challenges remain in terms of access to care and ongoing research efforts, the past to present ratio of neuronal disorders demonstrates a positive trend towards improved outcomes and a more compassionate approach to addressing these complex conditions. Neuronal disorders, also known as neurological disorders, encompass a wide range of conditions that affect the nervous system, including the brain, spinal cord, and peripheral nerves. These disorders can manifest in various ways, manifesting as cognitive decline, sensory dysfunction, or behavioral disturbances. Examples of neuronal disorders include Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, and stroke [3]. Etiological factors include genetic predispositions, environmental exposures, or multifactorial interactions. Treatment options for neuronal disorders vary depending on the specific condition and therapies such as medication and neurorehabilitation may be considered. Research in neuroscience continues to advance our understanding of these disorders, leading to improved diagnostic tools, treatment strategies, and potential cures in the future. In the meantime, if you are referring to preparations for managing or treating neuronal disorders, it typically involves a multidisciplinary approach. This may include consulting with neurologists, neuropsychologists, physical therapists, occupational therapists, and other healthcare professionals to develop a comprehensive treatment plan tailored to the individual’s specific needs. Medication management, therapy sessions, lifestyle modifications, and support from caregivers and family members are often key components of preparing for and managing neuronal disorders effectively [4]. Early diagnosis, regular monitoring, and adherence to treatment recommendations are crucial in optimizing outcomes for individuals living with neuronal disorders. Emerging neuropharmacological delivery platforms have enabled have revolutionized the treatment of neurological disorders by enabling targeted and efficient delivery of therapeutic agents to the brain and nervous system. One notable technique that has gained traction is the use of nanoparticles, liposomes, and other nanocarriers to encapsulate drugs and transport them across the blood-brain barrier, a protective barrier that restricts the passage of substances into the brain. These nanocarriers can be engineered to release drugs at specific locations within the brain, minimizing off-target effects and improving drug efficacy. Additionally, advancements in non-invasive drug delivery methods, such as focused ultrasound and nasal drug delivery, have shown promise in bypassing the blood-brain barrier and delivering drugs directly to the brain tissue. These innovative approaches hold great potential for enhancing the treatment of neurological disorders and improving patient outcomes in the future [5].
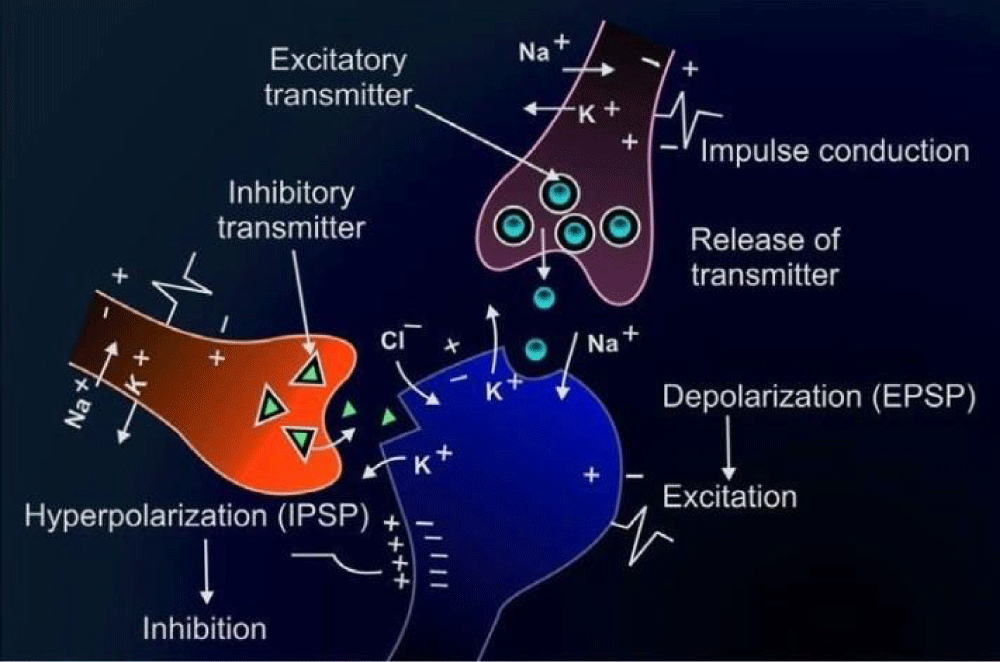
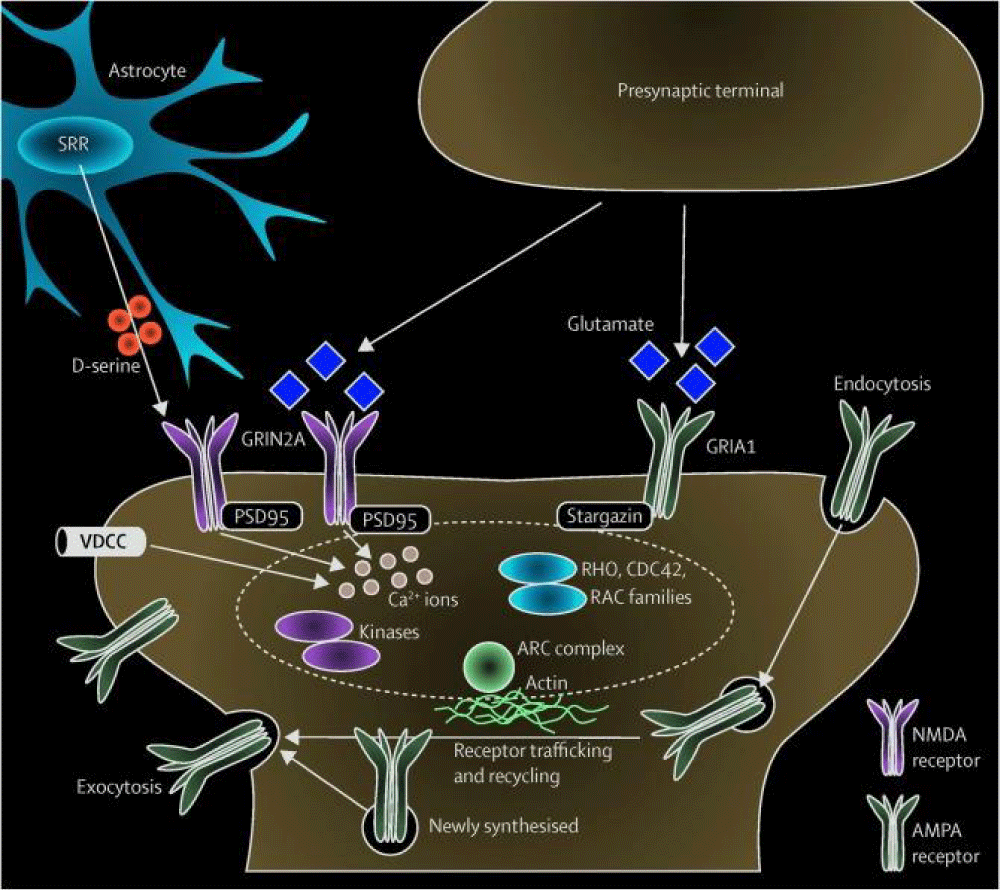
Pathogenesis of neurohumoral transmission
The pathogenesis of neurohumoral transmission involves the intricate interplay between the nervous system and various hormones or chemical messengers that regulate physiological processes in the body. Neurotransmitters, such as acetylcholine, dopamine, serotonin, and norepinephrine, are released by neurons in response to stimuli and play a crucial role in transmitting signals between nerve cells. These neurotransmitters bind to specific receptors on target cells, triggering a cascade of events that ultimately influence functions such as muscle contraction, mood regulation, and hormone secretion [6]. On the other hand, hormones produced by endocrine glands, such as the pituitary gland, adrenal glands, and thyroid gland, also play a significant role in regulating bodily functions. These hormones are released into the bloodstream and act on target organs or tissues to maintain homeostasis and respond to stressors. The pathogenesis of neurohumoral transmission involves the synthesis, release, and action of neurotransmitters and hormones, as well as the regulation of their levels in the body. Dysregulation of this intricate system can lead to various neurological and endocrine disorders, such as depression, anxiety, diabetes, and hormonal imbalances. Understanding the pathogenesis of neurohumoral transmission is essential for developing targeted therapies to treat these conditions and maintain overall health and well-being [7] (Figure 1).
Types of neuronal disorders
The diverse range of neuronal disorders that can affect the nervous system and impact an individual’s quality of life. Each disorder presents unique symptoms, causes, and treatment approaches, highlighting the complexity of neurological conditions.
Alzheimer’s disease
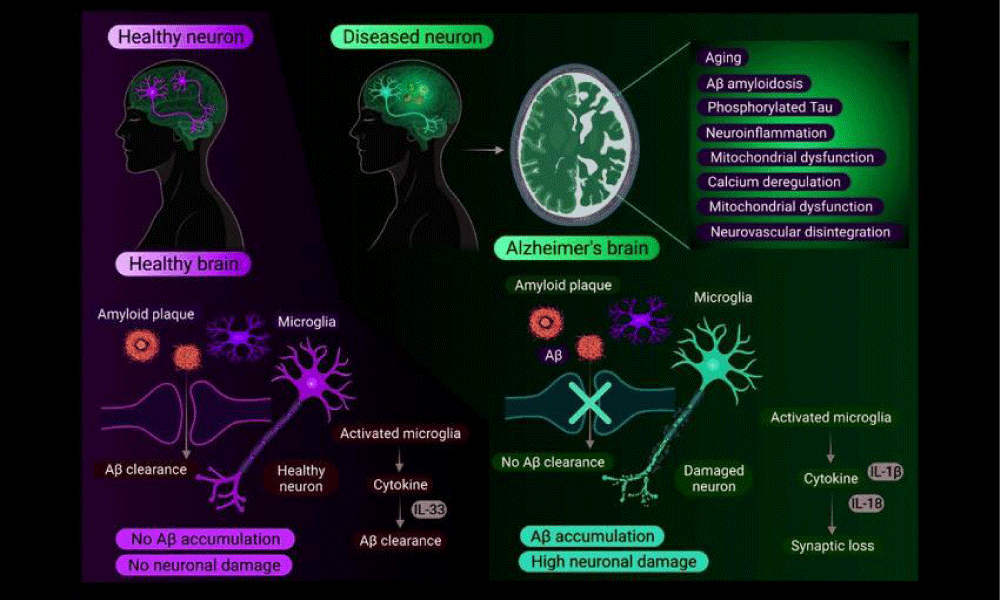
Alzheimer’s disease is a progressive neurodegenerative disorder that primarily affects memory, thinking, and behavior. It is the most common cause of dementia in older adults, marked by the accumulation of abnormal protein deposits in the brain, leading to the progressive neuronal loss and cognitive impairment. The early stages of Alzheimer’s disease may present with subtle symptoms, such as forgetfulness, difficulty in remembering recent events, and challenges in problem-solving or completing familiar tasks [8]. As the disease progresses, individuals may experience more severe symptoms, including confusion, disorientation, language difficulties, mood swings, and changes in personality. Timely identification and early-stage management are vital in managing Alzheimer’s disease and improving the quality of life for affected individuals. While there is no curative treatment exists to date for Alzheimer’s disease, treatments and interventions can help alleviate symptoms, slow down disease progression, and support overall well-being. Ongoing research in neuroscience aims to uncover new insights into the underlying mechanisms of Alzheimer’s disease and develop more effective therapies for this debilitating condition [9] (Figure 2).
Molecular pathogenesis: Alzheimer’s disease is a neurodegenerative disorder marked by the progressive loss of cognitive function and memory. The molecular pathogenesis of Alzheimer’s disease involves the accumulation of abnormal protein aggregates in the brain, particularly beta-amyloid plaques and tau tangles. Beta-amyloid plaques are formed from the aggregation of beta-amyloid peptides, which are derived from the cleavage of Amyloid Precursor Protein (APP). These plaques disrupt neuronal communication and lead to neuronal death [10]. Tau tangles, on the other hand, are formed from the hyperphosphorylation of tau protein, causing it to form twisted filaments inside neurons, disrupting their function. Additionally, inflammation, oxidative stress, and mitochondrial dysfunction play significant roles in the pathogenesis of Alzheimer’s disease. Understanding the molecular mechanisms underlying Alzheimer’s disease is crucial for developing effective treatments and interventions to slow down or prevent the progression of this devastating condition.
Parkinson’s disease
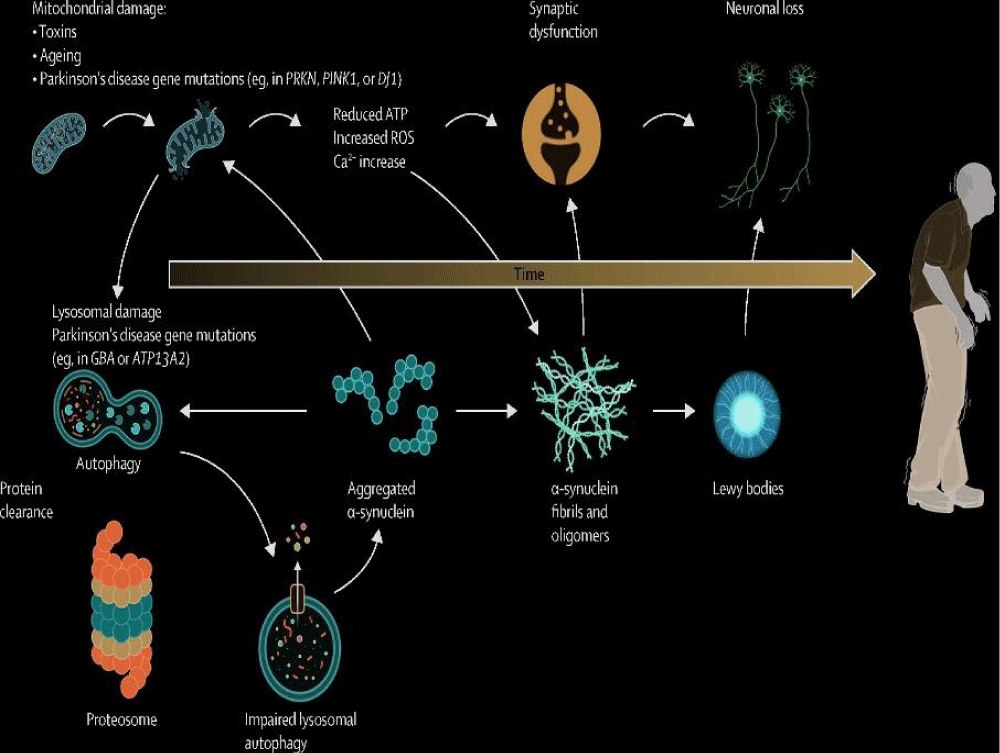
Parkinson’s disease is a neurodegenerative disorder that primarily affects movement. It is categorized by a progressive loss of dopamine-producing neurons in the brain, leading to symptoms such as tremors, bradykinesia (slowness of movement), rigidity, and postural instability [11]. In addition to motor symptoms, individuals with Parkinson’s disease may also experience non-motor symptoms such as cognitive impairment, depression, sleep disturbances, and autonomic dysfunction. The exact cause of Parkinson’s disease is not fully understood, but both genetic and environmental factors are believed to play a role. Early diagnosis and treatment can help manage symptoms and improve quality of life for individuals living with Parkinson’s disease (Figure 3).
Molecular pathogenesis: Parkinson’s disease is a neurodegenerative disorder portrayed by the progressive loss of dopaminergic neurons in the substantia nigra region of the brain [12]. The molecular pathogenesis of Parkinson’s disease involves a complex interplay of genetic and environmental factors. Genetic mutations, including those in SNCA, LRRK2, and Parkin genes have been implicated in familial forms of the disease, while environmental neurotoxins including certain pesticides and heavy metals can also contribute to its development. The accumulation of misfolded alpha-synuclein protein in Lewy bodies is a hallmark pathological feature of Parkinson’s disease. This aggregation leads to mitochondrial dysfunction, oxidative stress, and impaired protein degradation pathways, ultimately resulting in neuronal cell death. Understanding the molecular mechanisms underlying Parkinson’s disease is crucial for developing targeted therapies that can slow or halt disease progression. Ongoing research in this field aims to uncover novel therapeutic targets and improve the management of this debilitating condition [13].
Multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system, specifically the brain and spinal cord. It is depicted by the immune system mistakenly attacking the protective myelin sheath surrounding nerve fibers, leading to inflammation, demyelination, and scarring. The exact cause of MS is not fully understood, but it is believed to involve a combination of genetic, environmental, and immunological factors. The signs and symptoms of multiple sclerosis can vary widely depending on the location and extent of nerve damage. Symptoms vary but often include fatigue, limb numbness, and gait instability or tingling in the limbs, coordination and balance problems, vision disturbances, cognitive impairment, and bladder or bowel dysfunction. MS is typically diagnosed based on a combination of medical history, neurological examination, imaging tests (such as MRI), and laboratory tests. Early detection and treatment of multiple sclerosis are crucial in managing the disease and slowing its progression [14]. Treatment options may include disease-modifying medications to reduce inflammation and prevent relapses, symptom management therapies, physical therapy, and lifestyle modifications. It is clinically relevant for individuals with MS to work closely with healthcare providers to develop a personalized treatment plan that addresses their specific needs and improves their quality of life.
Molecular pathogenesis: Multiple sclerosis (MS) is a complex autoimmune disorder differentiated by inflammation, demyelination, and neurodegeneration in the central nervous system. The molecular pathogenesis of MS involves a dysregulated immune response targeting myelin, the protective sheath surrounding nerve fibers [15]. Immune cells, particularly T cells infiltrate the brain and spinal cord and trigger the release of pro-inflammatory cytokines and chemokines that promote inflammation and tissue damage. This inflammatory cascade disrupts the integrity of the blood-brain barrier, allowing immune cells to enter the central nervous system and attack myelin. Demyelination results in impaired nerve conduction and neuronal damage, leading to the clinical symptoms of MS, such as fatigue, weakness, sensory disturbances, and cognitive impairment. Genetic and environmental factors play a role in the development of MS, with certain genetic variants and viral infections implicated in disease susceptibility. Understanding the molecular mechanisms underlying MS pathogenesis is crucial for developing targeted therapies that modulate the immune response, promote remyelination, and preserve neuronal function in patients with MS (Figure 4).
Epilepsy
Epilepsy is a neurological disorder characterized by recurrent seizures, which are sudden, uncontrolled electrical disturbances in the brain. Seizures vary in type, severity, and duration depending on individual pathology, affecting a person’s consciousness, movements, sensations, or behavior. Epilepsy can develop at any age and may be caused by various factors, such as genetics, brain injury, infections, or developmental disorders. While epilepsy is a chronic condition, many individuals with epilepsy can effectively manage their seizures with medication, lifestyle changes, and in some cases, surgery. The signs and symptoms of epilepsy can vary widely among individuals and depend on the type of seizure experienced [16]. Common signs of epilepsy include sudden, unexplained staring spells, temporary confusion or loss of awareness, uncontrollable motor convulsions and auras presenting as déjà vu or fear or fear. Some individuals may experience auras, which are warning signs that a seizure is about to occur. It is essential to seek medical attention if you or someone you know experiences recurrent seizures or any of these symptoms, as early diagnosis and treatment are crucial in managing epilepsy effectively.
Molecular pathogenesis: Epilepsy is a neurological disorder characterized by recurrent seizures, which are caused by abnormal electrical activity in the brain. The molecular pathogenesis of epilepsy involves a complex interplay of genetic, environmental, and neurobiological factors. Mutations in genes encoding ion channels, neurotransmitter receptors, and synaptic proteins have been implicated in the development of epilepsy [17]. These genetic abnormalities can lead to alterations in neuronal excitability, synaptic transmission, and network connectivity, ultimately resulting in the generation of seizures. In addition to genetic factors, environmental insults including traumatic brain injury, neuroinfections, and exposure to toxins can also contribute to the pathogenesis of epilepsy [18]. Understanding the molecular mechanisms underlying epilepsy is crucial for the development of targeted therapies that can effectively control seizures and improve the quality of life for individuals living with this condition. Ongoing research in this field aims to uncover new therapeutic targets and treatment strategies to better manage epilepsy (Figure 5).
Stroke
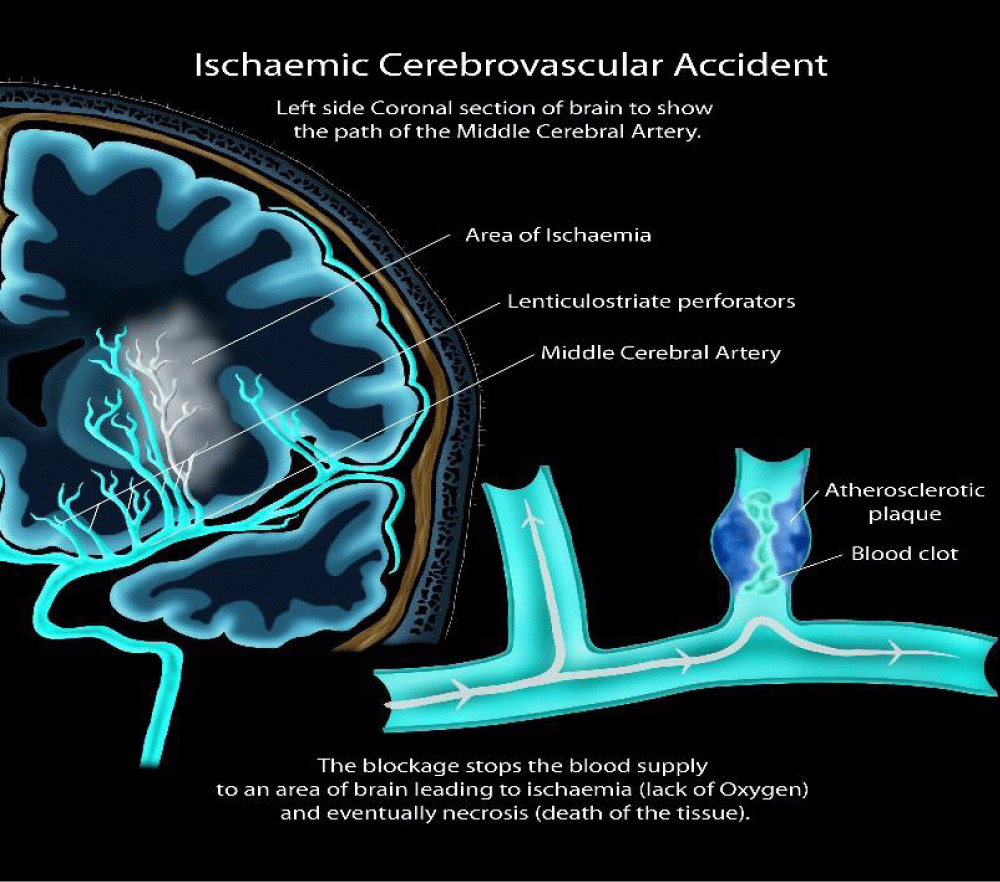
A stroke, also known as a Cerebrovascular Accident (CVA), occurs when there is a disruption in the blood supply to the brain, leading to damage or death of brain cells. Strokes can be classified into two main types: ischemic strokes, caused by a blockage in a blood vessel supplying the brain, and hemorrhagic strokes, caused by a rupture of a blood vessel in the brain. Strokes are a medical emergency and require immediate attention to minimize brain damage and prevent long-term disability [19]. The signs and symptoms of a stroke can vary depending on the type and location of the brain affected. Common signs of a stroke include acute hemiparesis or facial asymmetry, especially on one side of the body. Other symptoms may include sudden confusion, trouble speaking or understanding speech, difficulty walking, dizziness, and severe headache. It is clinically relevant to remember the acronym FAST (Face drooping, Arm weakness, Speech difficulty, Time to call emergency services) to recognize the signs of a stroke and seek immediate medical attention. Timely medical intervention significantly influences clinical prognosis in improving outcomes and reducing the risk of long-term disability associated with strokes [20].
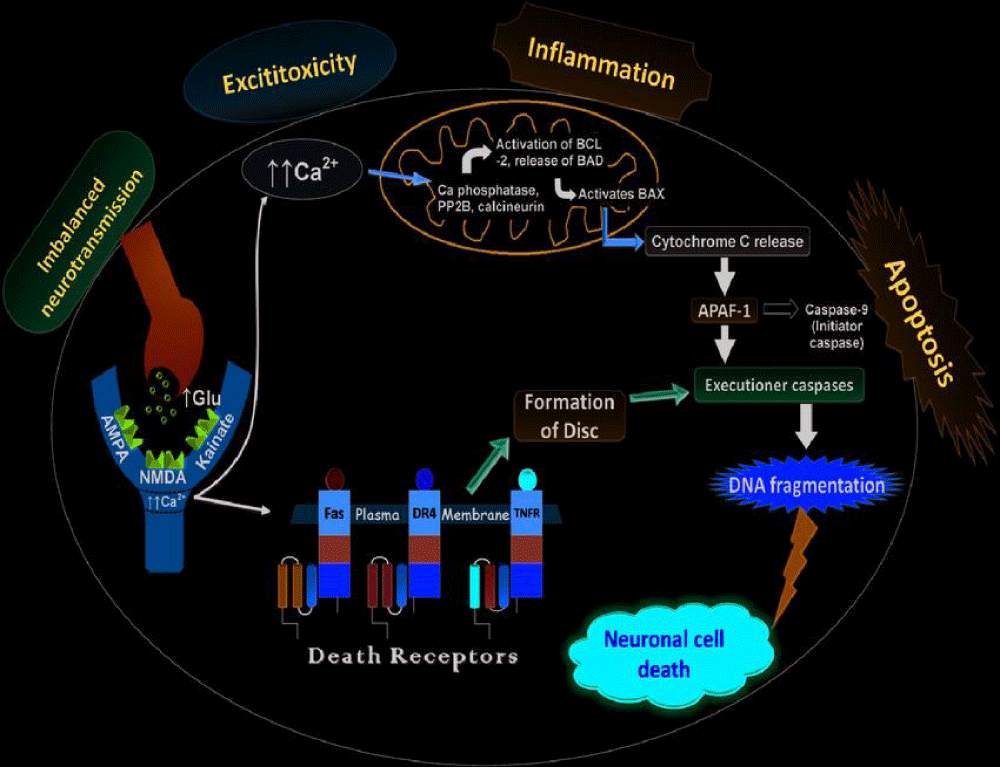
Molecular pathogenesis: Stroke is a complex medical condition that occurs when blood flow to the brain is disrupted, leading to damage or death of brain cells. The molecular pathogenesis of stroke involves a cascade of events that ultimately result in neuronal injury and tissue damage. One key mechanism is the interruption of blood supply, either due to a blockage (ischemic stroke) or a rupture (hemorrhagic stroke) of blood vessels in the brain. This leads to a decrease in oxygen and nutrient delivery to brain cells, triggering a series of molecular responses, including inflammation, oxidative stress, excitotoxicity, and apoptosis. These processes can further exacerbate brain damage and contribute to the development of secondary complications following a stroke. Understanding the molecular pathways involved in stroke pathogenesis is crucial for developing effective treatments and interventions to minimize brain injury and improve outcomes for stroke patients [21] (Figure 6).
Huntington’s disease
Huntington’s disease is a hereditary neurodegenerative disorder that affects the brain, leading to progressive physical, cognitive, and psychiatric symptoms. It is caused by a mutation in the HTT gene, which results in the production of a toxic protein that damages nerve cells in the brain. The disease commonly presents in mid-adulthood, although it can appear at any age. The early signs and symptoms of Huntington’s disease often include involuntary movements, such as jerking or twitching, as well as difficulties with coordination and balance. As the disease progresses, individuals may experience cognitive decline, including memory loss, impaired judgment, and difficulty concentrating. Psychiatric symptoms, such as depression, anxiety, irritability, and mood swings, are also common in individuals with Huntington’s disease. Early diagnosis and management of symptoms are notable in improving the quality of life for individuals with Huntington’s disease [22]. While there is no curative treatment exists to date for the condition, treatment options focus on symptom management and supportive care to help individuals maintain their independence and overall well-being. Genetic testing and counseling are recommended for individuals with a family history of Huntington’s disease to assess their risk and make informed decisions about their health.
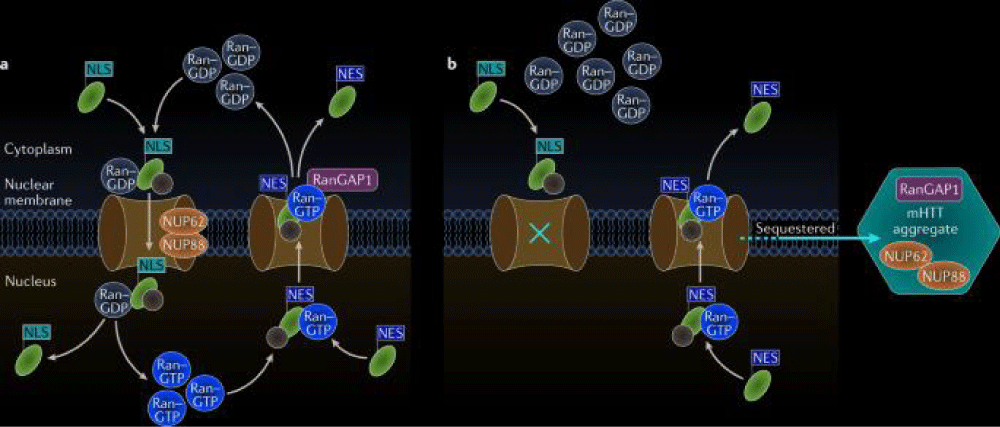
Molecular pathogenesis: Huntington’s disease is a neurodegenerative disorder caused by a mutation in the huntingtin gene, leading to the production of a mutant huntingtin protein. This abnormal protein accumulates in neurons, particularly in the striatum and cortex of the brain, disrupting cellular functions and ultimately causing neuronal death [23]. The molecular pathogenesis of Huntington’s disease involves a cascade of events, including mitochondrial dysfunction, oxidative stress, excitotoxicity, and impaired protein clearance mechanisms. These processes contribute to the progressive degeneration of neurons and the characteristic motor, cognitive, and psychiatric symptoms of the disease. Research into the molecular mechanisms underlying Huntington’s disease is ongoing, with the goal of developing targeted therapies to slow or halt disease progression (Figure 7).
Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder that affects the motor neurons in the brain and spinal cord. The exact cause of ALS is not fully understood, but it is believed to involve a combination of genetic and environmental factors. The onset of ALS is typically gradual, with initial symptoms including muscle weakness, twitching, and cramping [24]. As the disease progresses, individuals may experience difficulty walking, speaking, swallowing, and performing everyday tasks. Muscle atrophy and spasticity are common features of ALS, leading to a loss of motor function and eventual paralysis. Early diagnosis and intervention are essential in managing ALS symptoms and improving quality of life for patients. While there is no curative treatment exists to date for ALS, treatment options such as medication, physical therapy, and assistive devices can help alleviate symptoms and prolong survival. Research into the underlying mechanisms of ALS continues in the hopes of developing more effective therapies for this devastating disease [25].
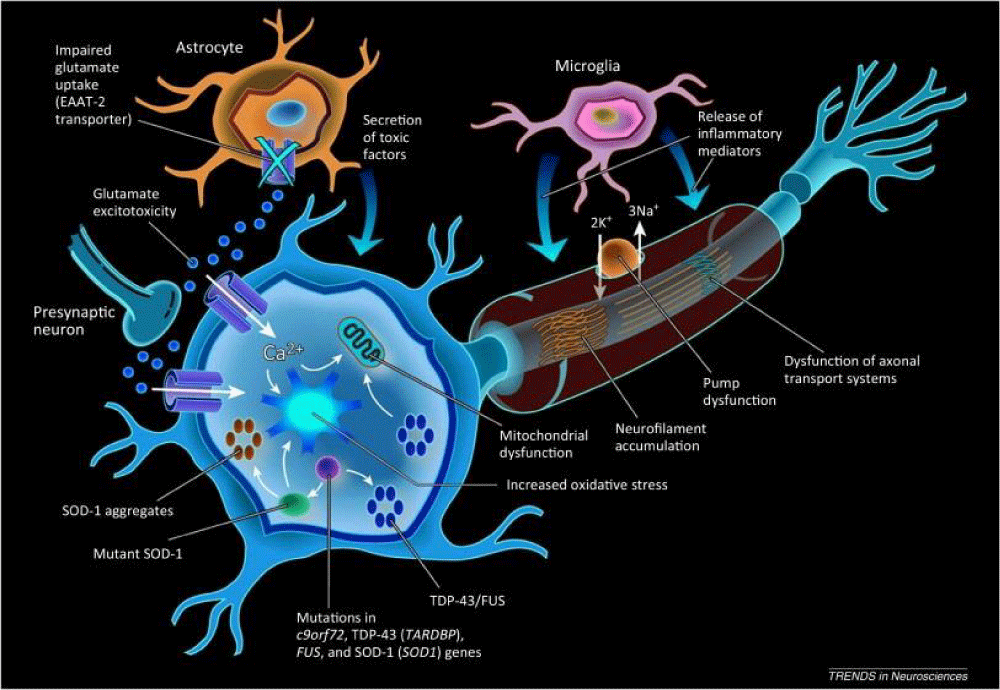
Molecular pathogenesis: Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder that results in the progressive degeneration of upper and lower motor neurons in the brain and spinal cord. The molecular pathogenesis of ALS is complex and involves multiple mechanisms that contribute to the degeneration of motor neurons. One key aspect of ALS pathogenesis is the accumulation of misfolded proteins, such as TDP-43 and SOD1, which form aggregates in motor neurons and disrupt cellular function. Additionally, abnormalities in RNA processing, mitochondrial dysfunction, oxidative stress, and neuroinflammation have been implicated in the pathogenesis of ALS. Genetic mutations in genes such as C9orf72, SOD1, FUS, and TARDBP have been identified in familial cases of ALS, highlighting the genetic basis of the disease. Understanding the molecular mechanisms underlying ALS is crucial for developing targeted therapies that can slow down or halt the progression of this devastating disease [26] (Figure 8).
Traumatic brain injury
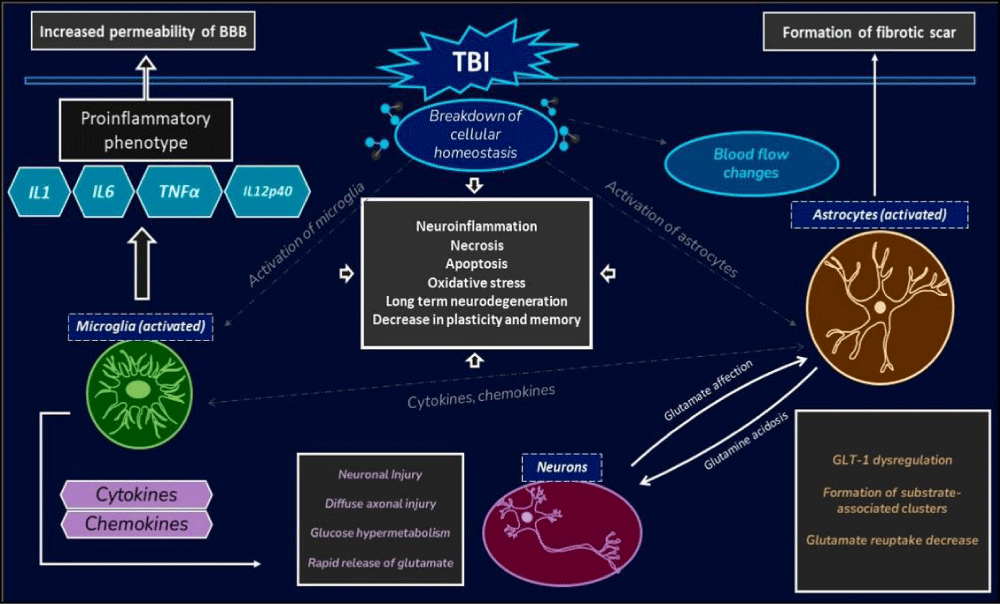
Traumatic Brain Injury (TBI) is a serious condition that occurs when a sudden trauma or blow to the head disrupts normal brain function. It can result from various incidents, such as falls, car accidents, sports injuries, or assaults. The severity of a TBI can range from mild (concussion) to severe, with potentially life-threatening consequences. The signs and symptoms of TBI can vary depending on the extent and location of the brain injury. Common symptoms of mild TBI include headaches, dizziness, confusion, memory problems, and changes in mood or behavior [27]. In more severe cases, individuals may experience loss of consciousness, seizures, slurred speech, weakness or numbness in the limbs, and profound cognitive deficits. It is crucial to seek immediate medical attention if someone exhibits any of these symptoms after a head injury, as prompt treatment can help prevent further damage and improve outcomes.
Molecular pathogenesis: Traumatic Brain Injury (TBI) is a complex condition that involves a cascade of molecular events leading to cellular damage and dysfunction in the brain. The molecular pathogenesis of TBI is characterized by primary injury, which occurs at the moment of impact, and secondary injury, which involves a series of biochemical and cellular processes that unfold in the hours and days following the initial trauma [28]. Primary injury results from mechanical forces causing direct damage to brain tissue, leading to cell death, blood-brain barrier disruption, and release of excitatory neurotransmitters. Secondary injury mechanisms include oxidative stress, inflammation, excitotoxicity, mitochondrial dysfunction, and apoptosis, which further exacerbate tissue damage and neuroinflammation. These molecular processes contribute to the progression of brain injury, leading to long-term neurological deficits and cognitive impairments. Understanding the molecular pathogenesis of TBI is crucial for developing targeted therapies to mitigate secondary injury mechanisms and improve outcomes for individuals affected by this condition (Figure 9).
Cerebral palsy
Cerebral palsy is a neurological disorder that affects movement, muscle tone, and posture. It is caused by damage to the developing brain, often before birth or during infancy. The condition is non-progressive, meaning that the brain injury does not worsen over time, but the symptoms can change as a child grows [29]. The signs and symptoms of cerebral palsy vary widely among individuals and can range from mild to severe. Common symptoms include muscle stiffness or floppiness, poor coordination, tremors, involuntary movements, and delays in reaching developmental milestones such as sitting up, crawling, or walking. Children with cerebral palsy may also experience difficulties with speech, vision, hearing, and cognitive function. Early detection and intervention are crucial in managing cerebral palsy and improving the quality of life for affected individuals. Treatment options may include physical therapy, occupational therapy, speech therapy, medications to manage symptoms, assistive devices, and surgery in some cases. A multidisciplinary approach involving healthcare professionals, educators, and caregivers is essential in providing comprehensive care for individuals with cerebral palsy [30].
Molecular pathogenesis: Cerebral palsy is a group of neurological disorders that affect movement and posture, often resulting from damage to the developing brain before, during, or shortly after birth. The molecular pathogenesis of cerebral palsy is complex and multifactorial, involving a combination of genetic and environmental factors. Recent research has focused on understanding the molecular mechanisms underlying the development of cerebral palsy, including inflammation, oxidative stress, excitotoxicity, and impaired neurodevelopment. These molecular interactions may result in disruptions in neuronal connectivity, brain development, and motor function, contributing to the symptoms observed in individuals with cerebral palsy [31]. Advances in molecular biology and genetics have provided insights into the pathogenesis of cerebral palsy, paving the way for potential therapeutic interventions and personalized treatment approaches in the future (Figure 10).
Tourette syndrome
Tourette syndrome is a neurodevelopmental disorder characterized by repetitive, involuntary movements and vocalizations known as tics. These tics can range from simple, such as eye blinking or throat clearing, to complex, such as jumping or repeating words or phrases. Symptoms of Tourette syndrome usually emerge during early childhood, with symptoms often peaking in the early teenage years. While the exact cause of Tourette syndrome is not fully understood, it is believed to involve a combination of genetic and environmental factors. Individuals with Tourette syndrome may also experience associated conditions such as Attention-Deficit/Hyperactivity Disorder (ADHD) and obsessive-compulsive disorder (OCD). Early diagnosis and intervention, including behavioral therapy and medication, can help manage symptoms and improve quality of life for individuals with Tourette syndrome [32].
Molecular pathogenesis: Tourette syndrome is a neurodevelopmental disorder characterized by repetitive, involuntary movements and vocalizations known as tics. The molecular pathogenesis of Tourette syndrome is complex and not fully understood. However, research suggests that genetic and environmental factors play a role in the development of the disorder. Studies have identified several genes that may be associated with Tourette syndrome, including those involved in neurotransmitter signaling, brain development, and immune function. Dysregulation of dopamine and other neurotransmitters in the brain has also been implicated in the pathogenesis of Tourette syndrome. Additionally, abnormalities in brain structure and function, particularly in regions involved in motor control and inhibition, have been observed in individuals with the disorder. Further research is needed to elucidate the precise molecular mechanisms underlying Tourette syndrome and to develop more effective treatments for this condition (Figure 11).
Autism spectrum disorder
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by challenges with social communication and interaction, as well as restricted and repetitive behaviors. Individuals with ASD may have difficulty with verbal and nonverbal communication, may demonstrate difficulty in affective reciprocity and emotional expression, exhibit repetitive movements or speech patterns, and show resistance to change in routines. Other common symptoms include sensory sensitivities, difficulties with social relationships, and intense focus on specific interests. Early diagnosis and intervention are crucial in managing symptoms and improving outcomes for individuals with ASD [33].
Molecular pathogenesis: The molecular pathogenesis of Autism Spectrum Disorder (ASD) is complex and not fully understood. Research suggests that genetic factors play a significant role in the development of ASD, with various genes implicated in the disorder. Mutations or variations in genes related to brain development, synaptic function, and neuronal communication have been identified in individuals with ASD. Additionally, environmental factors, such as prenatal exposure to certain substances or maternal infections, may also contribute to the development of ASD. Abnormalities in brain structure and function, including differences in connectivity between brain regions, have been observed in individuals with ASD. Further research is needed to fully elucidate the molecular mechanisms underlying ASD and to develop targeted treatments for the disorder [34] (Figure 12).
Schizophrenia
Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels, and behaves. It is characterized by a range of symptoms that can vary in severity and may include hallucinations, delusions, disorganized thinking, and incoherent speech, and impaired cognitive function. Individuals with schizophrenia may also experience social withdrawal, lack of motivation, and difficulty in expressing emotions. The onset of symptoms typically occurs in late adolescence or early adulthood, and the course of the disorder can be chronic with periods of relapse and remission. Early diagnosis and treatment, including medication and therapy, are essential in managing symptoms and improving outcomes for individuals with schizophrenia.
Molecular pathogenesis: The molecular pathogenesis of Schizophrenia is complex and multifactorial, involving a combination of genetic, environmental, and neurobiological factors. Research indicates that genetic predisposition plays a significant role in the development of schizophrenia, with multiple genes implicated in the disorder. Variations in genes related to neurotransmitter systems, synaptic function, and brain development have been associated with schizophrenia [35]. Additionally, environmental factors such as prenatal exposure to infections, stress, and substance abuse can increase the risk of developing schizophrenia. Abnormalities in brain structure and function, including alterations in neurotransmitter levels and connectivity between brain regions, have been observed in individuals with schizophrenia. Further research is needed to fully understand the molecular mechanisms underlying schizophrenia and to develop more effective treatments for the disorder (Figure 13).
Bipolar disorder
Bipolar disorder is a mental health condition characterized by extreme mood swings that include emotional highs (mania or hypomania) and lows (depression). Individuals with bipolar disorder may experience periods of elevated mood, increased energy, and impulsivity during manic episodes, followed by periods of sadness, fatigue, and hopelessness during depressive episodes. Other symptoms of bipolar disorder may include changes in sleep patterns, appetite, and concentration, as well as feelings of worthlessness or guilt. The onset of bipolar disorder typically occurs in late adolescence or early adulthood, and the disorder can have a significant impact on daily functioning and relationships. Treatment for bipolar disorder often involves a combination of medication, therapy, and lifestyle changes to help manage symptoms and stabilize mood [36].
Molecular pathogenesis: Bipolar disorder is a mental health condition characterized by extreme mood swings that include emotional highs (mania or hypomania) and lows (depression). The molecular pathogenesis of bipolar disorder is complex and involves a combination of genetic, environmental, and neurobiological factors. Research suggests that genetic predisposition plays a significant role in the development of bipolar disorder, with multiple genes implicated in the disorder. Neurotransmitter imbalances, particularly involving dopamine, serotonin, and norepinephrine, are also thought to contribute to the symptoms of bipolar disorder. Additionally, abnormalities in brain structure and function, including changes in the prefrontal cortex and limbic system, have been observed in individuals with bipolar disorder. Further research is needed to fully understand the molecular mechanisms underlying bipolar disorder and to develop more effective treatments for the condition (Figure 14).
Attention-deficit/hyperactivity disorder (ADHD)
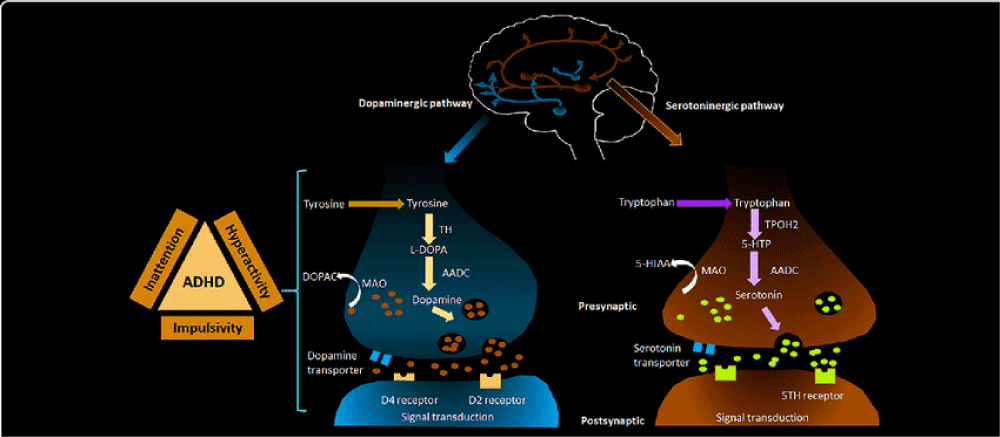
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder that affects both children and adults. It is characterized by persistent patterns of inattention, hyperactivity, and impulsivity that can interfere with daily functioning and social interactions. Individuals with ADHD may have difficulty sustaining attention, organizing tasks, following through on instructions, and controlling impulses. They may also exhibit hyperactive and impulsive behaviors, such as fidgeting, interrupting others, and acting without considering consequences. Symptoms of ADHD can vary in severity and may change over time. Early diagnosis and appropriate interventions, including behavioral therapy and medication, can help individuals with ADHD manage their symptoms and improve their quality of life [37].
Molecular pathogenesis: The molecular pathogenesis of Attention-Deficit/Hyperactivity Disorder (ADHD) is not fully understood, but research suggests that genetic and environmental factors play a role in the development of the disorder. Studies have identified several genes associated with ADHD, particularly those involved in neurotransmitter signaling, dopamine regulation, and brain development. Variations in these genes may contribute to differences in brain structure and function observed in individuals with ADHD. Additionally, environmental factors such as prenatal exposure to toxins, maternal smoking, or complications during pregnancy and birth have been linked to an increased risk of developing ADHD. Imbalances in neurotransmitters, particularly dopamine and norepinephrine, are thought to play a role in the symptoms of ADHD, including inattention, hyperactivity, and impulsivity. Further research is needed to better understand the molecular mechanisms underlying ADHD and to develop more targeted treatments for the disorder (Figure 15).
Obsessive-compulsive disorder (OCD)
Obsessive-Compulsive Disorder (OCD) is a mental health condition characterized by persistent, unwanted thoughts (obsessions) and repetitive behaviors or mental acts (compulsions). Individuals with OCD may experience intrusive thoughts or images that cause anxiety or distress, leading them to engage in compulsive behaviors to alleviate these feelings. Common obsessions include fears of contamination, doubts about safety, or a need for symmetry or order, while compulsions may involve repetitive actions like handwashing, checking, or counting. OCD can significantly impact daily functioning and quality of life if left untreated. Cognitive-behavioral therapy and medication are often used to manage symptoms and improve overall well-being for individuals with OCD [38].
Molecular pathogenesis: Obsessive-Compulsive Disorder (OCD) is a mental health condition characterized by intrusive, unwanted thoughts (obsessions) and repetitive behaviors or mental acts (compulsions) that are performed in response to these obsessions. The molecular pathogenesis of OCD is not fully understood, but research suggests that genetic and neurobiological factors play a role in the development of the disorder. Studies have identified abnormalities in the brain circuits involved in regulating fear, anxiety, and repetitive behaviors in individuals with OCD. Dysregulation of neurotransmitters such as serotonin, dopamine, and glutamate has also been implicated in the pathogenesis of OCD. Further research is needed to better understand the molecular mechanisms underlying OCD and to develop more effective treatments for the disorder.
Post-traumatic stress disorder (PTSD)
Post-Traumatic Stress Disorder (PTSD) is a mental health condition that can develop after experiencing or witnessing a traumatic event. Symptoms of PTSD can include intrusive memories of the traumatic event, flashbacks, nightmares, and severe emotional distress when reminded of the event [39]. Individuals with PTSD may also experience avoidance of situations or stimuli that remind them of the trauma, negative changes in mood or cognition, and heightened arousal or reactivity, such as irritability, hypervigilance, and difficulty sleeping. PTSD can significantly impact a person’s daily functioning and quality of life. Treatment for PTSD often involves therapy, medication, and support from mental health professionals. Early intervention is crucial in managing symptoms and improving outcomes for individuals with PTSD.
Molecular pathogenesis: Post-Traumatic Stress Disorder (PTSD) is a mental health condition that can develop after experiencing or witnessing a traumatic event. The molecular pathogenesis of PTSD involves complex interactions between genetic, neurobiological, and environmental factors. Research suggests that individuals with PTSD may have alterations in the stress response system, including dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis and abnormalities in neurotransmitter systems such as serotonin and dopamine. Changes in brain structure and function, particularly in regions involved in fear processing and emotional regulation, have also been observed in individuals with PTSD. Additionally, genetic factors may predispose some individuals to developing PTSD following trauma exposure. Understanding the molecular mechanisms underlying PTSD is crucial for developing targeted treatments and interventions to help individuals recover from the disorder [40].
Migraine
Migraine is a neurological condition characterized by recurrent episodes of moderate to severe headaches, often accompanied by other symptoms such as nausea, vomiting, and sensitivity to light and sound. Migraine headaches are typically pulsating or throbbing in nature and can last for hours to days. Some individuals may experience an “aura” before the onset of a migraine, which can include visual disturbances, sensory changes, or difficulty speaking. Migraines can significantly impact daily functioning and quality of life for those affected. Treatment options for migraines include medication to manage symptoms, lifestyle modifications, and stress management techniques. Early recognition of symptoms and appropriate management are key in effectively controlling migraines.
Molecular pathogenesis: Migraine is a neurological disorder characterized by recurrent episodes of moderate to severe headaches, often accompanied by other symptoms such as nausea, vomiting, and sensitivity to light and sound. The molecular pathogenesis of migraine involves complex interactions between genetic, environmental, and neurobiological factors. Research suggests that abnormalities in the brain’s neurotransmitter systems, particularly involving serotonin and dopamine, may play a role in the development of migraines. Additionally, changes in blood flow and vascular function in the brain have been implicated in migraine pathogenesis. Genetic studies have identified several gene variants associated with an increased risk of migraines. Further research is needed to fully understand the molecular mechanisms underlying migraines and to develop more effective treatments for this debilitating condition [41].
Restless legs syndrome
Restless Legs Syndrome (RLS) is a neurological disorder characterized by an irresistible urge to move the legs, often accompanied by uncomfortable sensations in the legs. Individuals with RLS may experience sensations such as tingling, crawling, or itching in the legs, which are typically worse at rest and improve with movement. Symptoms of RLS usually occur in the evening or at night, leading to difficulty falling asleep and disrupted sleep patterns. The exact cause of RLS is not fully understood, but factors such as genetics, iron deficiency, and certain medical conditions may contribute to the development of the disorder. Treatment for RLS may include lifestyle changes, medication, and therapy to help manage symptoms and improve quality of life.
Molecular pathogenesis: Restless Legs Syndrome (RLS) is a neurological disorder characterized by an irresistible urge to move the legs, often accompanied by uncomfortable sensations in the legs. The molecular pathogenesis of RLS is not fully understood, but research suggests that genetic factors may play a role in the development of the disorder. Mutations in genes related to iron metabolism, dopamine signaling, and neuronal excitability have been implicated in RLS. Additionally, abnormalities in brain regions involved in motor control and sensory processing have been observed in individuals with RLS. Imbalances in neurotransmitters, such as dopamine and glutamate, may also contribute to the symptoms of RLS. Further research is needed to better understand the molecular mechanisms underlying RLS and to develop targeted treatments for the disorder (Figure 16).
Narcolepsy
Restless Legs Syndrome (RLS) is a neurological disorder characterized by an irresistible urge to move the legs, often accompanied by uncomfortable sensations in the legs. Individuals with RLS may experience sensations such as tingling, crawling, or itching in the legs, which are typically worse at rest and improve with movement. Symptoms of RLS usually occur in the evening or at night, leading to difficulty falling asleep and disrupted sleep patterns. The exact cause of RLS is not fully understood, but factors such as genetics, iron deficiency, and certain medical conditions may contribute to the development of the disorder. Treatment for RLS may include lifestyle changes, medication, and therapy to help manage symptoms and improve quality of life [42].
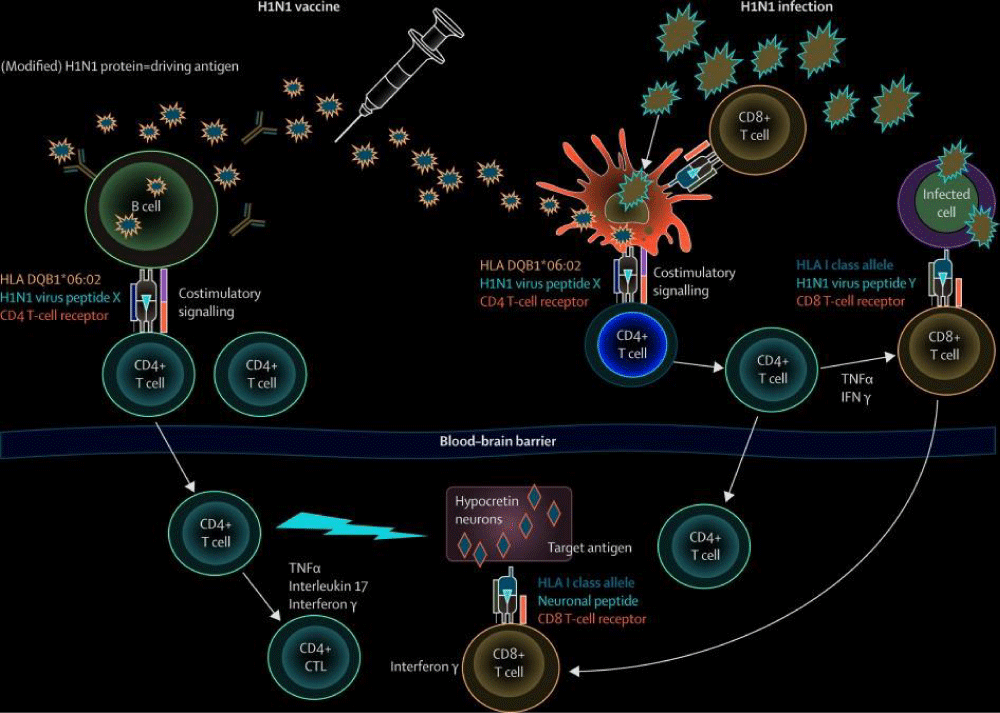
Molecular pathogenesis: Narcolepsy is a neurological disorder characterized by excessive daytime sleepiness, sudden loss of muscle tone (cataplexy), sleep paralysis, and hallucinations during sleep onset or upon awakening. The molecular pathogenesis of narcolepsy is primarily linked to the loss of hypocretin-producing neurons in the hypothalamus. Hypocretin, also known as orexin, is a neuropeptide that plays a crucial role in regulating wakefulness and sleep. In individuals with narcolepsy, autoimmune destruction of these neurons or genetic mutations affecting hypocretin signaling can lead to the symptoms of the disorder. Other factors, such as environmental triggers or infections, may also contribute to the development of narcolepsy. Understanding the molecular mechanisms underlying narcolepsy is essential for developing targeted therapies to manage the symptoms and improve the quality of life for individuals affected by the disorder [43] (Figure 17).
Peripheral neuropathy
Peripheral neuropathy is a condition that affects the peripheral nerves, which are responsible for transmitting signals between the central nervous system and the rest of the body. It can result from various causes, including diabetes, infections, autoimmune disorders, and exposure to toxins. Symptoms of peripheral neuropathy can vary depending on the type and location of the nerve damage but commonly include numbness, tingling, weakness, and pain in the affected areas. Individuals with peripheral neuropathy may also experience muscle cramps, sensitivity to touch, and changes in coordination and balance. Early diagnosis and management of underlying conditions are crucial in preventing further nerve damage and improving symptoms in individuals with peripheral neuropathy.
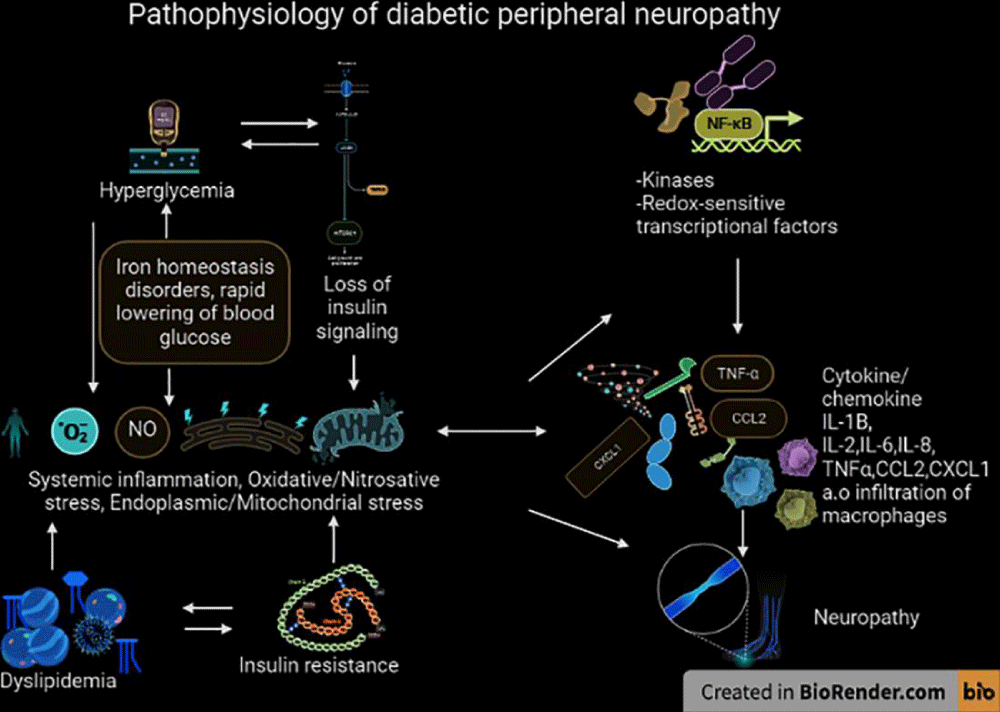
Molecular pathogenesis: Peripheral neuropathy refers to a condition in which the peripheral nerves, which transmit signals between the central nervous system and the rest of the body, become damaged or dysfunctional. The molecular pathogenesis of peripheral neuropathy can be caused by a variety of factors, including diabetes, autoimmune disorders, infections, exposure to toxins, and genetic predisposition. In diabetes, for example, high blood sugar levels can damage the nerves over time, leading to symptoms such as numbness, tingling, and weakness in the affected areas [44]. In autoimmune disorders, the immune system mistakenly attacks the nerves, causing inflammation and damage. Understanding the underlying molecular mechanisms of peripheral neuropathy is crucial for developing effective treatments and interventions to manage the condition and improve quality of life for affected individuals (Figure 18).
Drug delivery techniques
Nanoparticles for targeted drug delivery
Nanoparticles have shown great promise in the field of neuroscience for targeted drug delivery. The role of nanoparticles in this context includes.
- Enhanced blood-brain barrier penetration: Nanoparticles can be engineered to cross the blood-brain barrier, a protective barrier that restricts the passage of substances from the bloodstream into the brain. This allows for targeted delivery of drugs to specific regions of the brain, which is crucial for treating neurological disorders.
- Controlled release of drugs: Nanoparticles can be designed to encapsulate drugs and release them in a controlled manner. This controlled release profile can help maintain therapeutic drug levels in the brain over an extended period, improving treatment efficacy and reducing side effects.
- Targeted drug delivery to specific brain regions: By functionalizing nanoparticles with targeting ligands, researchers can direct the nanoparticles to specific cells or regions within the brain. This targeted approach minimizes off-target effects and enhances the therapeutic outcome of the drug [45].
- Imaging and diagnostic applications: Nanoparticles can also be used for imaging and diagnostic purposes in neuroscience. Functionalized nanoparticles can act as contrast agents in imaging techniques, allowing for the visualization of brain structures and abnormalities. Overall, nanoparticles offer a versatile platform for targeted drug delivery in neuroscience, enabling precise and effective treatment strategies for various neurological disorders.
Liposomes for encapsulation and controlled release
Liposomes have been extensively studied and utilized in neuroscience for their role in encapsulating and delivering drugs or therapeutic agents to the central nervous system. Liposomes confer multiple advantages in CNS-targeted pharmacotherapy.
- Targeted delivery: Liposomes can be engineered to target specific cells or tissues within the central nervous system, allowing for precise delivery of therapeutic agents to the desired site of action.
- Enhanced drug stability: Liposomes can protect labile therapeutic agents from enzymatic degradation and improve their stability, which is particularly relevant for drugs that are sensitive to enzymatic degradation in the brain.
- Controlled release: Liposomes can be designed to release their payload in a controlled manner, providing sustained release of drugs over an extended period of time. This controlled release profile can help maintain therapeutic drug levels in the brain and minimize potential side effects.
- Blood-brain barrier penetration: Liposomes can be modified to cross the blood-brain barrier, a protective barrier that restricts the entry of most drugs into the brain. By encapsulating drugs within liposomes, researchers can enhance their ability to penetrate the blood-brain barrier and reach the brain parenchyma. Overall, liposomes play a crucial role in neuroscience by facilitating the targeted delivery, controlled release, and enhanced stability of therapeutic agents for the treatment of various neurological disorders [46].
Microneedle patches for transdermal drug delivery
Microneedle patches for transdermal drug delivery in neuroscience offer a promising approach for delivering drugs across the skin barrier to target specific areas of the central nervous system. These systems employ micro-projections to breach the stratum corneum the outer layer of the skin, allowing for the controlled and targeted delivery of drugs directly into the bloodstream or underlying tissues. In the field of neuroscience, microneedle patches can be used to deliver neuroactive compounds, such as neurotransmitters, neuropeptides, or neuroprotective molecules directly to central nervous system targets. This targeted delivery method bypasses the blood-brain barrier, which can be a significant challenge in traditional drug delivery approaches for neurological disorders. Microneedle patches can also provide a non-invasive and painless alternative to traditional injection methods, reducing patient discomfort and improving compliance with treatment regimens. Additionally, the controlled release capabilities of microneedle patches allow for sustained drug delivery, minimizing fluctuations in drug levels and potentially improving therapeutic outcomes in neurological conditions. Overall, the role of microneedle patches for transdermal drug delivery in neuroscience is to enhance the precision, efficiency, and patient experience of drug administration for the treatment of various neurological disorders.
Implantable drug delivery systems
Implantable drug delivery systems play a crucial role in neuroscience by providing targeted and controlled delivery of therapeutic agents directly to the central nervous system. These systems offer several advantages in the field of neuroscience.
- Precise targeting: Implantable drug delivery systems can deliver drugs directly to specific regions of the brain or spinal cord, allowing for precise targeting of neural circuits involved in various neurological disorders.
- Controlled release: These systems can release drugs at a controlled rate over an extended period, ensuring a sustained therapeutic effect while minimizing systemic side effects.
- Minimized invasiveness: Implantable drug delivery systems can reduce the need for repeated injections or oral administration, thereby minimizing invasiveness and enhancing adherence and minimizing systemic exposure.
- Personalized treatment: These systems can be customized to deliver different drugs or drug combinations tailored to individual patient needs, offering personalized treatment options in neuroscience.
- Research tool: Implantable drug delivery systems are also valuable research tools for studying the effects of specific drugs on neural function and behavior in preclinical and clinical neuroscience studies. Overall, implantable drug delivery systems play a significant role in advancing our understanding of neurological disorders and developing targeted therapies for conditions such as Parkinson’s disease, epilepsy, chronic pain, and neurodegenerative diseases [47].
Inhalable drug delivery devices
Inhalable drug delivery devices play a significant role in neuroscience by providing a non-invasive and efficient method of delivering drugs to the brain. These devices allow for the direct administration of drugs to the respiratory system, where they can quickly enter the bloodstream and reach the brain through the blood-brain barrier. Inhalable drug delivery devices are particularly useful in the field of neuroscience for several reasons:
- Targeted delivery: Inhalable devices can deliver drugs directly to the brain, bypassing other organs and minimizing systemic side effects.
- Rapid onset of action: Inhalable drugs can reach the brain quickly, leading to faster therapeutic effects compared to other routes of administration.
- Improved patient compliance: Inhalable devices offer improved patient tolerability and non-invasive administration benefits than other methods of drug delivery, leading to better patient acceptance and adherence to treatment regimens.
- Precise dosing: Inhalable devices allow for precise control over drug dosage, ensuring that the right amount of medication reaches the brain. Overall, inhalable drug delivery devices offer a promising approach for the treatment of neurological disorders and have the potential to improve patient outcomes in the field of neuroscience.
Hydrogels for sustained release
Hydrogels have shown promise in the field of neuroscience for sustained drug delivery applications. The role of hydrogels in neuroscience includes.
- Controlled release: Hydrogels can be engineered to release drugs or therapeutic agents in a controlled and sustained manner, ensuring sustained therapeutic levels over an extended period of the drug at the target site in the brain or nervous system. This controlled release can help minimize side effects and improve treatment efficacy.
- Localized delivery: Hydrogels can be designed to deliver drugs directly to specific regions of the brain or spinal cord, bypassing the blood-brain barrier and reducing systemic exposure. This targeted delivery can enhance the therapeutic effects of drugs while minimizing off-target effects.
- Biocompatibility: These hydrophilic polymeric matrices are inherently biocompatible that are well-tolerated by the body, making them suitable for implantation in neural tissues. This biocompatibility nature reduces the risk of inflammation or rejection reactions, making hydrogels a safe option for sustained drug delivery in neuroscience applications [48].
- Tunable properties: Hydrogels can be tailored to have specific properties such as porosity, degradation rate, and mechanical strength, allowing researchers to customize the delivery system based on the requirements of the drug and the target tissue in the nervous system. Overall, hydrogels offer a versatile platform for sustained drug delivery in neuroscience, providing a promising approach for the treatment of neurological disorders and injuries.
Microfluidic devices for precise dosing
Microfluidic devices play a crucial role in neuroscience by providing a platform for precise dosing and controlled delivery of drugs or compounds to study the effects on neural cells or tissues. These devices offer several advantages in neuroscience research.
- Precise control: Microfluidic devices allow researchers to precisely control the flow rate, concentration, and timing of drug delivery to neural cells or tissues. Such control enables precise modulation of exposure and response for studying the effects of drugs on neuronal activity, synaptic transmission, and neuroplasticity.
- High throughput screening: Microfluidic devices enable high throughput screening of multiple drugs or compounds simultaneously on neural cells or tissues. This allows researchers to facilitating high-throughput pharmacological screening for neurological disorders or study the mechanisms of action of different compounds.
- Mimicking in vivo conditions: Microfluidic devices can be designed to mimic the complex microenvironment of the brain, including the presence of multiple cell types, gradients of signaling molecules, and physical barriers. This enables researchers to study drug effects in a more physiologically relevant context.
- Long-term studies: Microfluidic devices can support long-term culture of neural cells or tissues, allowing researchers to study the chronic effects of drugs on neuronal function, survival, and connectivity over extended periods. Overall, microfluidic devices provide a powerful tool for studying the effects of drugs on neural cells and tissues in a controlled and precise manner, advancing our understanding of neurobiology and potential therapeutic interventions for neurological disorders.
Gene therapy for targeted delivery of genetic material
Gene therapy plays a significant role in neuroscience by offering a promising approach for targeted delivery of genetic material to treat various neurological disorders. In the context of neuroscience, gene therapy can be applied to transfer corrective gene constructs to target cells to specific cells in the brain or nervous system to restore functional gene expression by correcting genetic mutations, restore normal gene function, or modulate gene expression [49]. Some key roles of gene therapy for targeted delivery of genetic material in neuroscience include:
- Treatment of genetic neurological disorders: Gene therapy can be used to deliver functional genes to replace mutated or defective genes responsible for inherited neurological disorders such as Huntington’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS).
- Modulation of gene expression: Gene therapy can be employed to regulate the expression of specific genes involved in neurodegenerative diseases, neurodevelopmental disorders, or brain injuries, offering potential therapeutic benefits.
- Targeted delivery to specific brain regions: Gene therapy techniques can be tailored to target specific cell types or brain regions, allowing for precise delivery of therapeutic genes to the affected areas while minimizing off-target effects.
- Neuroprotection and neuroregeneration: Gene therapy approaches can promote neuroprotection by enhancing cell survival, reducing inflammation, or preventing neuronal degeneration. Additionally, gene therapy strategies can stimulate neuroregeneration by promoting the growth and repair of damaged neurons in the central nervous system. Overall, gene therapy for targeted delivery of genetic material in neuroscience holds great promise for developing innovative treatments for a wide range of neurological disorders, offering the potential to address the underlying genetic causes and provide personalized therapeutic interventions for patients.
3D printing for personalized drug delivery systems
3D printing for personalized drug delivery systems in neuroscience plays a crucial role in advancing treatment options for neurological disorders. By utilizing 3D printing technology, researchers and healthcare professionals can create customized drug delivery systems tailored to individual patient needs. This personalized approach allows for precise dosing, customized implants enabling precision drug localization within brain regions, and improved therapeutic outcomes. In the field of neuroscience, 3D printing can be used to create intricate structures such as implants, scaffolds, or microdevices that can deliver drugs directly to the brain or spinal cord. These personalized drug delivery systems may facilitate bypassing the blood-brain barrier through spatially customized designs, a major challenge in treating neurological conditions, and enhance the efficacy of drug therapies. Overall, the role of 3D printing for personalized drug delivery systems in neuroscience is to revolutionize treatment strategies, improve patient outcomes, and pave the way for more effective and targeted therapies for neurological disorders [50].
Bioadhesive drug delivery systems for localized treatment
Bioadhesive drug delivery systems play a crucial role in neuroscience by providing targeted and localized treatment for neurological disorders [51]. These systems adhere to neural mucosa or perivascular regions to enhance localized retention, such as the blood-brain barrier, allowing for sustained release of therapeutic agents directly to the affected area. In neuroscience, bioadhesive drug delivery systems offer several advantages:
- Targeted delivery: Bioadhesive systems can target specific regions of the brain, allowing for precise delivery of drugs to the affected area while minimizing systemic side effects [52].
- Enhanced drug efficacy: By maintaining a high concentration of the drug at the site of action, bioadhesive systems can improve the efficacy of neuroactive compounds and enhance therapeutic outcomes.
- Prolonged drug release: These platforms offer mucoadhesive properties enabling prolonged site-specific drug retention over an extended period, ensuring a constant supply of medication to the brain and reducing the frequency of dosing [53].
- Protection of therapeutic agents: Bioadhesive systems can protect sensitive drugs from degradation and metabolism, increasing their stability and bioavailability in the brain. Overall, bioadhesive drug delivery systems offer a promising approach for the treatment of neurological disorders by providing targeted and localized delivery of therapeutic agents to the brain. Further research and development in this area hold great potential for improving the management of various neurological conditions [54].
Conclusion
Neurons are the fundamental building blocks of the nervous system, responsible for transmitting electrical and chemical signals throughout the body. They play a crucial role in processing information, controlling movement, regulating bodily functions, and forming memories. Neurons consist of a cell body, dendrites, and an axon, which work together to transmit signals through a process known as synaptic transmission. Neurons communicate with each other through specialized connections called synapses, where neurotransmitters are released to transmit signals from one neuron to another. This intricate network of neurons forms the basis of our thoughts, emotions, and behaviors. Understanding the basics of neurons is essential for comprehending how the brain functions and how neurological disorders can impact our cognitive abilities and overall well-being. By studying neurons and their interactions, researchers can gain insights into brain function and develop treatments for neurological conditions. In the past, neuroscience has made significant strides in understanding the complexities of the brain and nervous system. Technological advancements, including neuroimaging, electrophysiology, and molecular biology have allowed researchers to unravel the mysteries of the brain and advance our knowledge of brain function and behavior. With technological convergence, neuroscience is expected to evolve to continue its rapid advancement with the integration of cutting-edge technologies such as artificial intelligence, machine learning, and optogenetics. These emerging tools are enabling deeper exploration of neural networks the brain at unprecedented levels of detail and complexity, leading to new discoveries and insights into brain function and neurological disorders. Overall, the future of neuroscience holds great promise for furthering our understanding of the brain and developing innovative treatments for neurological conditions. The integration of bioengineering, nanomedicine, and computational modeling continues to redefine therapeutic approaches and interdisciplinary approaches, neuroscience is poised to significantly advance neurobiological research and therapeutic innovation and improve the neurological health and quality of life in affected individuals by neurological disorders. In the present, advancements in nanotechnology and drug delivery systems have significantly improved the efficiency and precision of delivering drugs to the brain. Nanoparticles can be engineered to target specific brain regions or cells, allowing for more targeted and effective drug delivery. Additionally, new techniques such as focused ultrasound and implantable devices have been developed enabling localized CNS drug administration while minimizing systemic exposure and procedural invasiveness. Overall, the field of neuro drug delivery has made significant progress in overcoming the challenges of delivering drugs to the brain. These innovations offer substantial potential for improving the treatment of neurological disorders and optimizing pharmacokinetics and therapeutic outcomes in CNS disorders.
Portions of this manuscript, including language refinement, structural reorganization, and stylistic improvements, were developed with the assistance of ChatGPT (OpenAI, Version 4, 2025). The AI tool was used solely to enhance clarity and academic tone, and did not contribute to the scientific content, interpretation of data, or generation of original research material. All content was reviewed and edited by the authors, who assume full responsibility for the integrity and accuracy of the manuscript.
- Ross LN, Bassett DS. Causation in neuroscience: keeping mechanism meaningful. Nat Rev Neurosci. 2024;25(2):81-90. Available from: https://doi.org/10.1038/s41583-023-00778-7
- Nau M, Schmid AC, Kaplan SM, Baker CI, Kravitz DJ. Centering cognitive neuroscience on task demands and generalization. Nat Neurosci. 2024 Jul 29:1-2. Available from: https://doi.org/10.1038/s41593-024-01711-6
- Liu S, Wang L, Gao RX. Cognitive neuroscience and robotics: advancements and future research directions. Robot Comput Integr Manuf. 2024;85:102610. Available from: https://doi.org/10.1016/j.rcim.2023.102610
- Sahu MK, Tiwari SP. Role of nanomaterials in pharmaceutical preparation: a review. Int J Nanomater Nanotechnol Nanomed. 2024;10(2):056-67. Available from: https://www.chemisgroup.us/articles/IJNNN-10-163.pdf
- Badrulhisham F, Pogatzki-Zahn E, Segelcke D, Spisak T, Vollert J. Machine learning and artificial intelligence in neuroscience: a primer for researchers. Brain Behav Immun. 2024;115:470-9. Available from: https://doi.org/10.1016/j.bbi.2023.11.005
- Sahu MK, Tiwari SP. Phytochemical and ethnopharmacological review of Aegle marmelos. Bull Pioneer Res Med Clin Sci. 2024;3(2):29-47. Available from: https://bprmcs.com/article/phytochemical-and-ethnopharmacological-review-of-aegle-marmelos-8uudyskewiwmjpr
- Bzdok D, Thieme A, Levkovskyy O, Wren P, Ray T, Reddy S. Data science opportunities of large language models for neuroscience and biomedicine. Neuron. 2024;112(5):698-717. Available from: https://doi.org/10.1016/j.neuron.2024.01.016
- Sahu MK, Tiwari SP. Epidemiology, pathogenesis and treatment of diabetes: a comprehensive review. Wor Jour Dia Res Pract. 2024;1(1):1-9. Available from: https://mkscienceset.com/articles_file/573-_article1708434044.pdf
- Lindsay GW. Grounding neuroscience in behavioral changes using artificial neural networks. Curr Opin Neurobiol. 2024;84:102816. Available from: https://doi.org/10.1016/j.conb.2023.102816
- Singh B. Evolutionary global neuroscience for cognition and brain health: strengthening innovation in brain science. In: Biomedical Research Developments for Improved Healthcare. IGI Global; 2024. p. 246-72. Available from: http://dx.doi.org/10.4018/979-8-3693-1922-2.ch012
- Sahu MK. A review on glaucoma: causes, symptoms, pathogenesis & treatment. J Clin Res Ophthalmol. 2024;11(1):001-4. Available from: https://www.clinsurggroup.us/articles/JCRO-11-202.pdf
- Hyde LW, Bezek JL, Michael C. The future of neuroscience in developmental psychopathology. Dev Psychopathol. 2024 Mar 6:1-6. Available from: https://doi.org/10.1017/s0954579424000233
- Kaur V. Neurostrategy: a scientometric analysis of marriage between neuroscience and strategic management. J Bus Res. 2024;170:114342. Available from: https://doi.org/10.1016/j.jbusres.2023.114342
- Sahu MK, Nayak AK, Hailemeskel B, Eyupoglu OE. Exploring recent updates on molecular docking: types, method, application, limitation & future prospects. Int J Pharm Res Allied Sci. 2024;13(2):24-40. Available from: https://ijpras.com/article/exploring-recent-updates-on-molecular-docking-types-method-application-limitation-amp-future-p-xqlkubawc6eu8xl
- Ocklenburg S, Güntürkün O. The lateralized brain: the neuroscience and evolution of hemispheric asymmetries. Amsterdam: Elsevier; 2024. Available from: https://shop.elsevier.com/books/the-lateralized-brain/ocklenburg/978-0-323-99737-9
- Barksdale BR, Doss MK, Fonzo GA, Nemeroff CB. The mechanistic divide in psychedelic neuroscience: an unbridgeable gap? Neurotherapeutics. 2024 Jan 25:e00322. Available from: https://doi.org/10.1016/j.neurot.2024.e00322
- Montag C, Ali R, Davis KL. Affective neuroscience theory and attitudes towards artificial intelligence. AI Soc. 2024 Jan 28:1-8. Available from: https://link.springer.com/article/10.1007/s00146-023-01841-8
- Hayashi S, Caron BA, Heinsfeld AS, Vinci-Booher S, McPherson B, Bullock DN, et al. brainlife.io: a decentralized and open-source cloud platform to support neuroscience research. Nat Methods. 2024;21(5):809-13. Available from: https://doi.org/10.1038/s41592-024-02237-2
- Sahu B, Sahu M, Sahu M, Yadav M, Sahu R, Sahu C. An updated review on Nelumbo nucifera Gaertn: chemical composition, nutritional value and pharmacological activities. Chem Biodivers. 2024 Feb 7:e202301493. Available from: https://doi.org/10.1002/cbdv.202301493
- Nelson CA, Sullivan E, Engelstad AM. Annual research review: early intervention viewed through the lens of developmental neuroscience. J Child Psychol Psychiatry. 2024;65(4):435-55. Available from: https://doi.org/10.1111/jcpp.13858
- Keshavan MS, Song SH, Zhang Y, Lizano P. Neuroscience in pictures: 1. History of psychiatric neuroscience. Asian J Psychiatr. 2024;92:103869. Available from: https://doi.org/10.1016/j.ajp.2023.103869
- Imaniyati N, Ramdhany MA, Rasto R, Nurjanah S, Solihah PA, Susilawati A. Neuroscience intervention for implementing digital transformation and organizational health completed with literature review, bibliometrics, and experiments. Indones J Sci Technol. 2024;9(2):287-336. Available from: http://dx.doi.org/10.17509/ijost.v9i2.67763
- Cao R, Yamins D. Explanatory models in neuroscience, Part 2: functional intelligibility and the contravariance principle. Cogn Syst Res. 2024;85:101200. Available from: https://doi.org/10.1016/j.cogsys.2023.101200
- Buccella A, Maoz U, Mudrik L. Towards an interdisciplinary “science of the mind”: a call for enhanced collaboration between philosophy and neuroscience. Eur J Neurosci. 2024 Jul 2. Available from: https://doi.org/10.1111/ejn.16451
- Xu J, Yang Y, Huang D, Gururajapathy SS, Ke Y, Qiao M, et al. Data-driven network neuroscience: on data collection and benchmark. Adv Neural Inf Process Syst. 2024;36. Available from: https://doi.org/10.48550/arXiv.2211.12421
- Han H. Considering the purposes of moral education with evidence in neuroscience: emphasis on habituation of virtues and cultivation of phronesis. Ethical Theory Moral Pract. 2024;27(1):111-28. Available from: https://philarchive.org/rec/HYECTP
- Kansra P, Oberoi S, Gupta SL, Singh N. Factors limiting the application of consumer neuroscience: a systematic review. J Consum Behav. 2024;23(1):31-42. Available from: http://dx.doi.org/10.1002/cb.2131
- Fischer H, Nilsson ME, Ebner NC. Why the single-N design should be the default in affective neuroscience. Affect Sci. 2024;5(1):62-6. Available from: https://doi.org/10.1007/s42761-023-00182-5
- Carneiro de Lima da Silva AL, Cabral Seixas Costa AP, de Almeida AT. Analysis of the cognitive aspects of the preference elicitation process in the compensatory context: a neuroscience experiment with FITradeoff. Int Trans Oper Res. 2024;31(4):2472-503. Available from: https://doi.org/10.1111/itor.13210
- Clark PJ, Brodnik ZD, España RA. Chemogenetic signaling in space and time: considerations for designing neuroscience experiments using DREADDs. Neuroscientist. 2024;30(3):328-46. Available from: https://doi.org/10.1177/10738584221134587
- Sahu MK, Yadav R, Tiwari SP. Recent advances in nanotechnology. Int J Nanomater Nanotechnol Nanomed. 2023;9(1):015-23. Available from: https://www.chemisgroup.us/articles/IJNNN-9-153.php
- Mahendra Kumar S. Development and preclinical evaluation of anti-analgesic, anti-pyretic, anti-inflammatory and antioxidant potential of polyherbal formulation on experimental animal model. Int J Cell Sci Mol Biol. 2023;7(3):555716. Available from: https://juniperpublishers.com/ijcsmb/pdf/IJCSMB.MS.ID.555716.pdf
- Mancusi R, Monje M. The neuroscience of cancer. Nature. 2023;618(7965):467-79. Available from: https://doi.org/10.1038/s41586-023-05968-y
- Sahu MK, Tiwari SP. A systemic review on recent outbreaks of dengue and newer US FDA approved drug. J Infect Dis Virus Res. 2023;2(2):01-6. Available from: https://mkscienceset.com/articles_file/575-_article1687167234.pdf
- Mahendra KS, Mandavi G, Dushika S, Surendra S. Recent challenges and opportunities on Middle East Respiratory Syndrome Corona Virus (MERS-CoV). Vaccines Vaccin. 2023;8(1):000154. Available from: https://medwinpublishers.com/VVOA/recent-challenges-and-opportunities-on-middle-east-respiratory-syndrome-corona-virus-(mers-cov).pdf
- Drammeh I, Yadav R, Sahu MK. Exploring uterine fibroids and its treatment in current scenario. IP Int J Compr Adv Pharmacol. 2023;8(3):143-8. Available from: https://doi.org/10.18231/j.ijcaap.2023.025
- Winkler F, Venkatesh HS, Amit M, Batchelor T, Demir IE, Deneen B, et al. Cancer neuroscience: state of the field, emerging directions. Cell. 2023;186(8):1689-707. Available from: https://doi.org/10.1016/j.cell.2023.02.002
- Sahu MK, Dubey N, Pandey R, Shukla SS, Gidwani B. Formulation, evaluation, and validation of microspheres of cyclophosphamide for topical delivery. Pharmacophore. 2023;14(1):1-8. Available from: https://doi.org/10.51847/e4GvuoN96z
- Nebe S, Reutter M, Baker DH, Bölte J, Domes G, Gamer M, et al. Enhancing precision in human neuroscience. Elife. 2023;12:e85980. Available from: https://doi.org/10.7554/elife.85980
- Sterratt D, Graham B, Gillies A, Einevoll G, Willshaw D. Principles of computational modelling in neuroscience. Cambridge University Press; 2023 Oct 5. Available from: https://assets.cambridge.org/97805218/77954/frontmatter/9780521877954_frontmatter.pdf
- Levenstein D, Alvarez VA, Amarasingham A, Azab H, Chen ZS, Gerkin RC, et al. On the role of theory and modeling in neuroscience. J Neurosci. 2023;43(7):1074-88. Available from: https://doi.org/10.1523/jneurosci.1179-22.2022
- Sahu MK, Saraf S. Convection-enhanced delivery of alkaloid-loaded maghemite nanoparticles against 9L gliomass cell line. Adv Pharm Clin Trials. 2022;7(4):000210. Available from: https://doi.org/10.23880/apct-16000210
- Collins S. Neuroscience for learning and development: How to apply neuroscience and psychology for improved learning and training. London: Kogan Page Publishers; 2023 Jun 3.
- Gkintoni E, Dimakos I, Halkiopoulos C, Antonopoulou H. Contributions of neuroscience to educational praxis: A systematic review. Emerg Sci J. 2023 Jul 27;7:146-58. Available from: https://www.ijournalse.org/index.php/ESJ/article/view/1717
- Kumar A, Kumar D, Kumar R, Prasad J, Kumar M, Joshi P, et al. Peptic ulcers and their complications. J Drug Deliv Ther. 2020 Jun 15;10(3-s):256-61. Available from: https://jddtonline.info/index.php/jddt/article/view/4082
- Sahu MK, Satapathy T, Netam AK, Prasad J. Structural architecture and signal transduction of ion channels: A review. Res J Pharmacol Pharmacodyn. 2018;10(1):38-44. Available from: https://doi.org/10.5958/2321-5836.2018.00007.1
- Satapathy T, Kaushik S, Netam AK, Prasad J, Rao SP, Sahu MK, et al. Nano delivery: a smart carrier for treatment of ovarian cancer. [Journal name and link missing – please provide]
- Singh VK, Sahu MK. Antidiabetic medicinal plants having insulin mimetic property: A review. Kenkyu J Pharm Pract Health Care. 2018;60:56-60.
- Prasad J, Netam AK, Singh R, Sahu M, Satapathy T, Rao SP, et al. Therapeutic approaches for the management of Parkinson's disease. Res J Pharmacol Pharmacodyn. 2019;11(1):46-52. Available from: https://doi.org/10.5958/2321-5836.2019.00009.0
- Sahu MK, Singh VK, Rao SP. Development and evaluation of antidiabetic potential of polyherbal formulation in streptozotocin induced animal model. Int J Cell Sci Mol Biol. 2018;5(2):0029-37. Available from: https://juniperpublishers.com/ijcsmb/pdf/IJCSMB.MS.ID.555656.pdf
- Jain S, Saraf S, Sahu MK. Recent trends and strategies for targeting M-cells via oral vaccine against hepatitis B: A review. Int J Cell Sci Mol Biol. 2019;5(5):86-92. Available from: https://ideas.repec.org/a/adp/ijcsmb/v5y2019i5p86-92.html
- Sahu AK, Satapathy T, Kumar R, Joshi P, Kumar M, Singh R, Prasad J, Latkar J. Recovery from addiction: a strong will but rare success. [Journal name and link missing – please provide]
- Kriegeskorte N, Douglas PK. Cognitive computational neuroscience. Nat Neurosci. 2018;21(9):1148-60. Available from: https://doi.org/10.1038/s41593-018-0210-5
- Prasad J, Netam AK, Sahu MK, Satapathy T. Current concepts in clinical-based management of diabetic foot infections: A review. Res J Pharmacol Pharmacodyn. 2017;9(3):157-66. Available from: https://doi.org/10.5958/2321-5836.2017.00027.1